
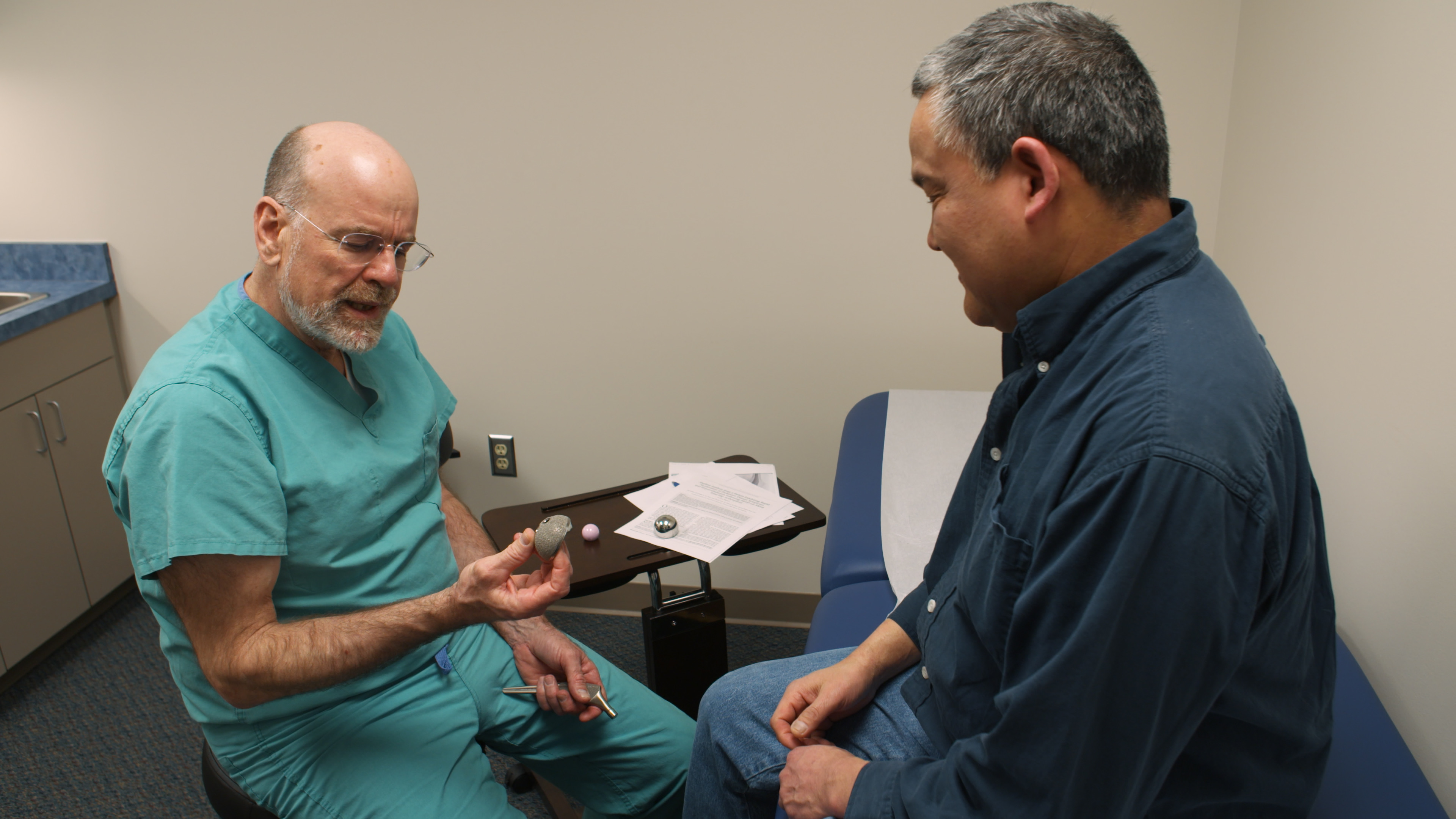
“Some patients implanted with the Essure System for Permanent Birth Control have experienced and/or reported adverse events, including perforation of the uterus and/or fallopian tubes, identification of inserts in the abdominal or pelvic cavity, persistent pain, and suspected allergic or hypersensitivity reactions. In its statement last week, Bayer said it “is reminding women with Essure that the safety profile of the device remains positive and unchanged.” Immergut called Essure “an innovative Class III medical device” that was approved under the FDA’s Premarket Approval (PMA) review, “the agency’s most rigorous pathway for medical devices.”īayer’s website for Essure currently has a prominent safety-information warning about the device: This week, Fuentes set up a GoFundMe page to raise funds for living expenses and has received $5,830 in donations so far according to Fuentes, she has found a place to live thanks to a homeless-assistance program and has been reunited with her children. Her medical complications eventually prevented her from working, and she was forced to place her four daughters in foster homes. In “The Bleeding Edge,” one Essure user who is profiled, Ana Fuentes, describes excruciating abdominal pain and chronic bleeding after she had the device implanted in 2011.
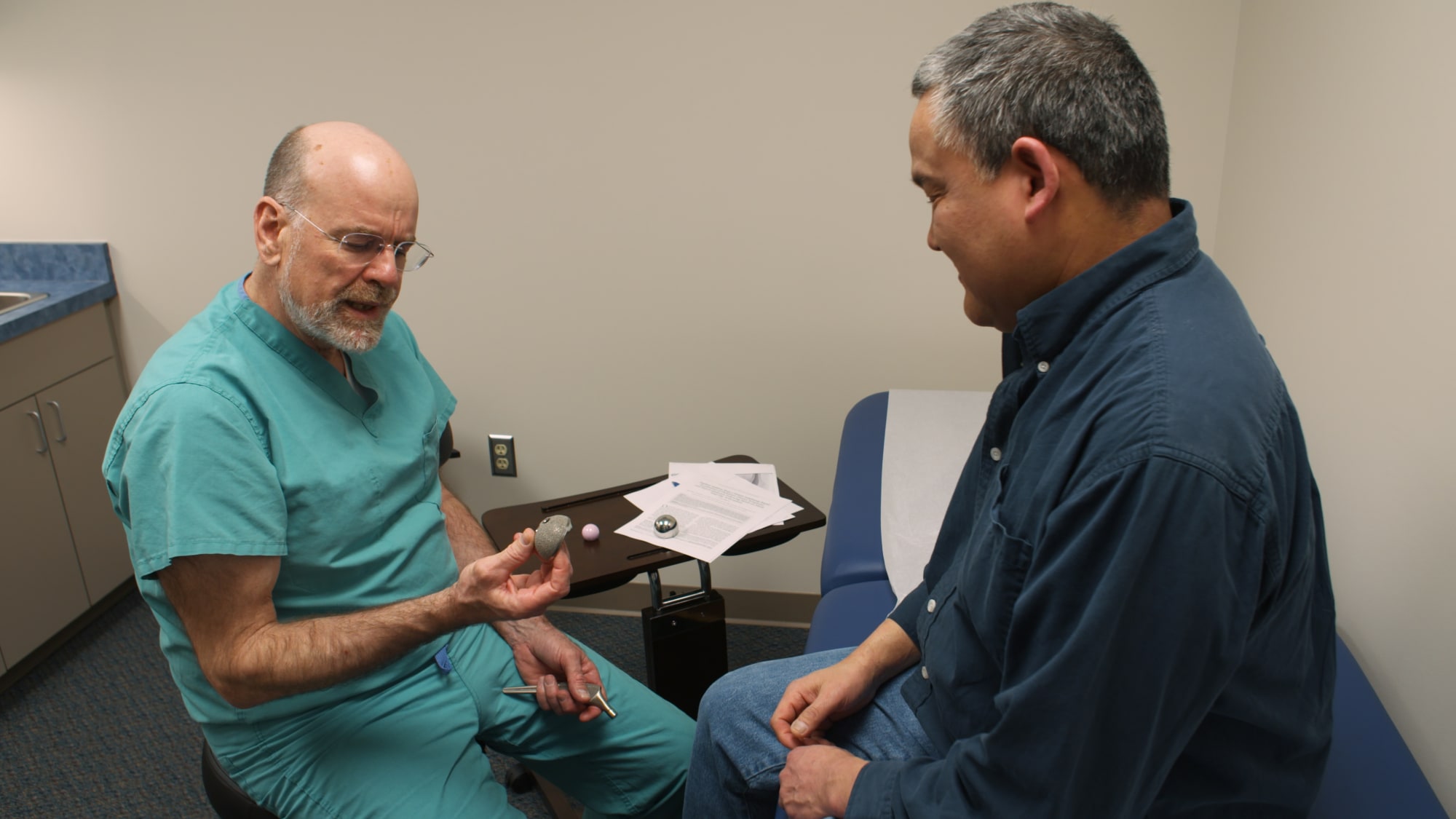
According to a rep for “The Bleeding Edge” filmmakers, Netflix supports their statement. Netflix has not directly weighed in on the controversy. The company also is being sued in Canada, having been served with two lawsuits over Essure that are seeking class-action certification. “Plaintiffs allege personal injuries from the use of Essure, including hysterectomy, perforation, pain, bleeding, weight gain, nickel sensitivity, depression and unwanted pregnancy, and seek compensatory and punitive damages,” Bayer said in the Q1 report. lawsuits representing approximately 16,800 users of Essure as of April 13, 2018. The pharma giant said it anticipates additional lawsuits in the U.S. In Bayer’s first-quarter 2018 earnings report, the company said it had been served with U.S.
#Bleeding edge netflix trial
“These ethical concerns are among the reasons why FDA did not require such a trial for Essure.”

That “would clearly not be possible or ethical for a surgical procedure like laparoscopic tubal ligation or the permanent placement of Essure,” Immergut said. Moreover, according to Bayer, a blinded, randomized clinical trial requires that neither the patient nor the physician knows which treatment the patient is receiving. “‘The Bleeding Edge’ producers’ comment that Essure trials ‘weren’t randomized or non-blinded’ and… ‘lacked comparator groups’ only further exposes their ignorance of the underlying science and the ethics involved in studying a medical device, like Essure, and demonstrates the deficiencies of their documentary,” Steven Immergut, VP, head of communications, pharmaceuticals for Bayer U.S., said in a statement sent late Friday to Variety.Īccording to Immergut, the suggestion that Essure should have been put through randomized clinical trials “completely ignores the ethical problems with creating a comparison group of women who were randomly subjected to invasive laparoscopic tubal ligation surgery, the only alternative permanent birth control for women.” 3), Bayer issued a response to the filmmakers’ statements.


 0 kommentar(er)
0 kommentar(er)
